Table of Contents
Introduction: The Scientific Journey of Peaches Through Time

A peach stone (fossilized Peach fruit) found in Zhejiang Province tells a story eight thousand years old. The fossilized remnant, discovered by archaeologists in eastern China, marks one of humanity’s earliest encounters with Prunus persica. What began as a bitter, small-fruited tree in mountain valleys has become a global symbol of summer sweetness. Yet beneath the velvety skin and dripping juice lies something more remarkable: a genetic manuscript recording millennia of evolutionary change.
Modern molecular tools have transformed how scientists read this manuscript. Through genome sequencing and comparative genetics, researchers can now trace the exact mutations that turned astringent wild fruits into dessert-quality peaches. They can identify the genes responsible for fragrance compounds that vary from region to region. They can pinpoint the chromosomal changes that allowed peaches to flower earlier, grow fuzz, and accumulate sugar at concentrations wild ancestors never achieved. This article explores six major genetic secrets that shaped peaches from their origins in northwestern China through the Silk Road dispersal and into contemporary orchards worldwide. Each secret reveals how evolution, human selection, and environmental pressure collaborated to create the fruit we recognize today.
Comparison of Peaches with Other Rosaceae Fruits
| Fruit | Key Traits Compared to Peaches |
|---|---|
| Peaches | Soft, fuzzy skin; high sucrose-to-acid ratio; strong lactone aroma; medium perishability. |
| Apples | Firmer flesh; lower sugar; longer storage life; less aromatic. |
| Cherries | Smaller, juicier; higher anthocyanin content; more tartness. |
| Pears | Grainier texture; higher sorbitol; less acidity and aroma diversity. |
| Apricots | Smaller and denser; similar stone fruit genetics; earlier ripening. |
| Plums | Higher phenolic content; deeper pigmentation; variable sweetness. |
| Strawberries | Aggregate fruit; non-stone type; strong volatile profile; shorter shelf life. |
| Raspberries | Hollow drupelets; high fiber; delicate skin; distinct floral esters. |
1. Peaches and Their Wild Ancestor: The Legacy of Prunus davidiana
The evolutionary lineage of cultivated peaches traces back to wild relatives that still grow in mountainous regions of Tibet and western China. Among these, Prunus davidiana played a crucial genetic role. This wild species contributed genes for cold hardiness that allowed early peach varieties to survive harsh winters. Genomic comparisons show that cultivated peaches inherited chromosomal segments from P. davidiana containing stress response genes.
The split between wild and domesticated peaches occurred roughly four to five million years ago at the species level. But the domestication event itself happened around six thousand years ago in the Yellow River valley. During this transition, ancient farmers selected trees with larger fruits and reduced bitterness. However, they unknowingly preserved certain wild traits. Genes controlling drought tolerance and pathogen resistance remained embedded in the cultivated genome. These genetic remnants prove invaluable today as breeders seek to reintroduce resilience into modern varieties that have lost some ancestral toughness through intensive selection for palatability.
Whole genome sequencing completed in 2013 revealed that peaches experienced a severe genetic bottleneck during domestication. This bottleneck reduced genetic diversity by approximately forty percent compared to wild populations. The narrowing occurred because early cultivators propagated only a small number of superior individuals through grafting. Despite this reduction, enough variation persisted to generate the thousands of cultivars grown worldwide. Researchers now use wild Prunus species as genetic reservoirs to broaden the limited diversity of commercial peaches.
Wild Ancestor Contributions to Cultivated Peaches
| Genetic Trait | Source and Function |
|---|---|
| Cold tolerance genes | Inherited from P. davidiana populations in high-altitude regions |
| Disease resistance loci | Maintained from wild ancestors despite domestication bottleneck |
| Root architecture genes | Contributed by wild species adapted to rocky mountain soils |
| Drought stress response | Retained from ancestors in semi-arid northwestern Chinese habitats |
| Phenolic compound production | Wild lineages produced higher levels for herbivore defense |
| Flowering time variation | Wild populations showed broader range allowing local adaptation |
| Seed dispersal mechanisms | Ancestral traits largely eliminated in domesticated forms |
| Genetic diversity markers | Wild populations contain 1.7 times more SNP variation |
2. Peaches and the Sweetness Code: Sugar Gene Selection
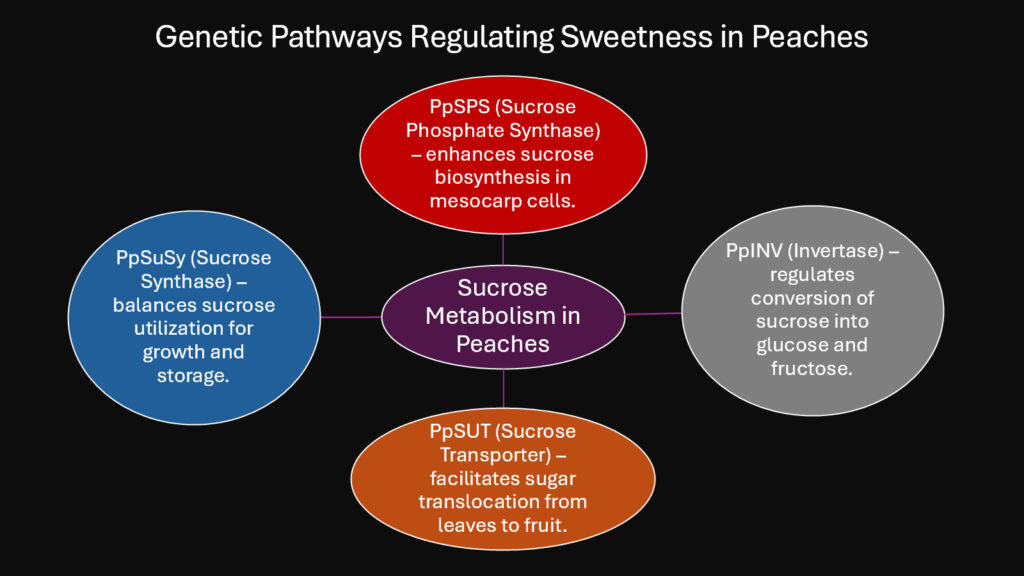
Sweetness defines the modern peach experience, but wild ancestors contained far less sugar. The transformation from tart to sweet involved specific genetic changes in sugar metabolism pathways. Peaches accumulate three main sugars: sucrose, glucose, and fructose. The relative proportions and total concentrations vary dramatically among cultivars. Some varieties reach twenty percent sugar by weight, while others remain below ten percent.
Two gene families govern this variation. Sucrose phosphate synthase genes, particularly PpSPS1, regulate the final steps of sucrose production. Increased expression of this gene leads to higher sweetness scores. Conversely, invertase genes like PpINV break down sucrose into component sugars. Cultivars with lower invertase activity maintain higher sucrose levels and taste sweeter. Ancient Chinese and Persian breeders selected for sweetness without understanding these molecular mechanisms. They simply chose the tastiest fruits for propagation.
Modern genetic studies using CRISPR technology have confirmed which genes matter most. When researchers knocked out specific invertase genes, resulting fruits accumulated significantly more sucrose. Similarly, overexpressing SPS genes boosted total sugar content. These experiments validate what traditional breeding accomplished through trial and error over millennia. The sugar gene complex occupies specific chromosomal regions that show clear signatures of selection. These genomic regions have reduced variation because the same favorable alleles were selected repeatedly across different breeding programs.
Sugar accumulation also depends on source-sink relationships within the tree. Leaves produce sugars through photosynthesis, then transport them to developing fruits. Transporter genes control this movement. Cultivars with enhanced transporter expression deliver more sugars to fruits, resulting in sweeter taste. The entire process involves coordination between dozens of genes acting in leaves, stems, and fruit tissues. This genetic orchestra creates the signature sweetness that made peaches desirable enough to spread from China across three continents.
Genetic Basis of Sweetness in Peaches
| Component | Genetic Regulation |
|---|---|
| Sucrose synthesis | Controlled primarily by PpSPS1 gene with high selection signatures |
| Glucose and fructose ratio | Regulated by invertase gene family including PpINV variants |
| Sugar transporters | SWEET gene family moves sugars from leaves to fruits |
| Sugar accumulation timing | Developmental genes activate synthesis during fruit ripening |
| Acid-sugar balance | Organic acid genes interact with sugar genes for taste perception |
| Starch to sugar conversion | Amylase genes break down starch in mature fruits |
| Metabolic regulation | Transcription factors coordinate entire sugar pathway activation |
| Selection intensity | Sugar loci show 30-40% reduced heterozygosity from breeding |
3. Peaches and Aroma Evolution: The Volatile Gene Pathway
The distinctive peach fragrance comes from over one hundred volatile organic compounds. Among these, gamma-decalactone provides the characteristic creamy, peachy note. Beta-ionone contributes floral and woody undertones. These molecules form through branched biosynthetic pathways starting from fatty acids and carotenoids. Genetic variations in pathway enzymes create the aroma diversity found across cultivars.
Lactone synthesis depends on fatty acid metabolism. Enzymes called lipoxygenases initiate the process by oxidizing unsaturated fatty acids. Downstream enzymes then convert these oxidized products into lactones through complex reactions. A specific gene encoding acyl-CoA oxidase appears critical for gamma-decalactone production. Cultivars with higher expression of this gene produce more intense peach aroma. Chinese varieties often emphasize different volatile profiles than European or American types, reflecting centuries of independent selection.
The genetics of aroma proved more complex than sweetness. Unlike sugar genes with clear major effects, aroma involves many genes with smaller individual contributions. Quantitative trait locus mapping identified at least fifteen chromosomal regions affecting volatile production. These regions contain genes for terpene synthases, alcohol dehydrogenases, and methyltransferases. Each enzyme modifies precursor molecules into different aromatic compounds. Mutations changing enzyme specificity or expression levels alter the final fragrance bouquet.
Regional climates also influenced aroma evolution. Mediterranean peaches developed different volatile profiles than temperate Asian varieties because environmental conditions affected which aromas provided selective advantages. Higher temperatures increase volatile emission rates, potentially attracting different seed dispersers. Human preferences further shaped aroma genetics as people in different regions selected fruits matching local taste traditions. This dual selection from environment and culture created the geographic mosaic of peach fragrances found today.
Volatile Compounds and Their Genetic Control
| Aroma Compound | Biosynthetic Pathway and Genes |
|---|---|
| Gamma-decalactone | Produced via fatty acid pathway, key gene is acyl-CoA oxidase |
| Beta-ionone | Derived from carotenoid breakdown by carotenoid cleavage dioxygenase |
| Linalool | Synthesized by terpene synthase genes from geranyl pyrophosphate |
| Hexanal and hexanol | Generated through lipoxygenase pathway from linoleic acid |
| Benzaldehyde | Formed from phenylalanine by enzymes in phenylpropanoid pathway |
| Ethyl acetate | Produced by alcohol acyltransferase combining ethanol and acetyl-CoA |
| Eugenol | Synthesized through phenylpropanoid pathway, rare in most cultivars |
| Terpenoid diversity | Controlled by gene family of 30+ terpene synthase variants |
4. Peaches and Early Flowering: A Genetic Shortcut to Survival
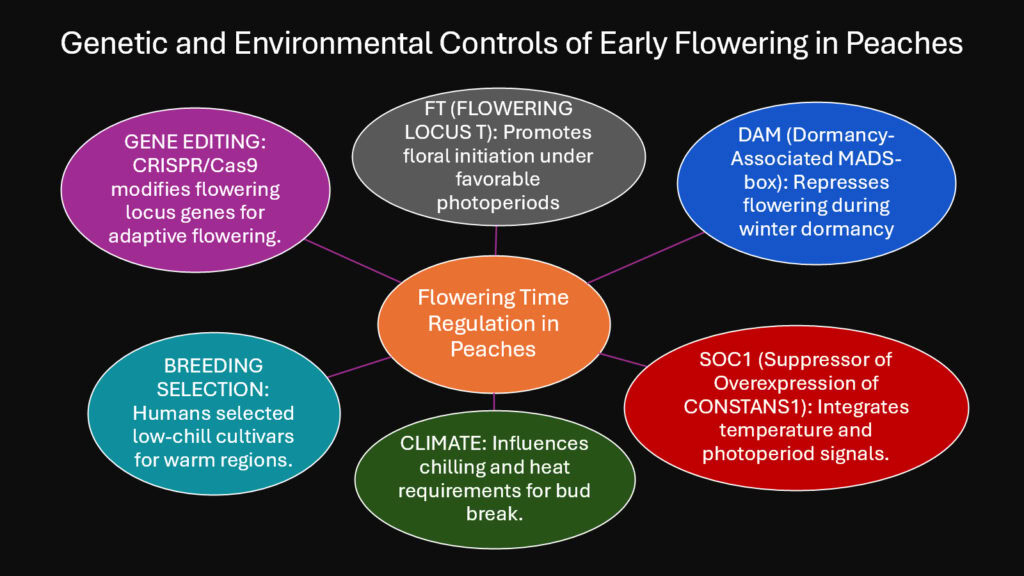
Flowering time represents one of the most critical adaptations in peach evolution. Trees must flower late enough to avoid frost damage but early enough to ripen fruits before unfavorable conditions return. This narrow window drove selection for precise flowering control. Peaches evolved genetic mechanisms allowing them to track seasonal changes and respond accordingly.
The FLOWERING LOCUS T gene family controls when peaches initiate flowering. This gene integrates signals about temperature and day length. When conditions match genetic programming, FT genes trigger the switch from vegetative growth to flower production. Peaches also possess DORMANCY ASSOCIATED MADS-box genes that regulate winter dormancy. These DAM genes must be properly silenced before flowering can begin. Low temperatures during winter gradually deactivate DAM genes through epigenetic modifications.
Different cultivars carry different alleles of flowering genes, explaining why some varieties bloom weeks earlier than others. Southern peach cultivars selected for mild winters have weaker dormancy requirements. They carry DAM gene variants that require fewer chilling hours for deactivation. Northern varieties possess stronger dormancy controlled by different DAM alleles. This genetic variation allowed peaches to colonize diverse climate zones as they spread from China.
Climate change now poses challenges to this finely tuned system. Warmer winters provide insufficient chilling in some regions, preventing proper DAM gene silencing. Trees then flower erratically or not at all. Breeders are responding by identifying low-chill alleles from subtropical varieties and incorporating them into new cultivars. Understanding the genetic basis of flowering time enables rational breeding strategies to maintain productivity under shifting climate conditions.
Flowering Time Genetic Architecture
| Genetic Component | Function and Variation |
|---|---|
| FT gene family | Integrates environmental signals to initiate flowering transition |
| DAM genes | Six copies regulate dormancy depth and chilling requirement |
| Chilling requirement | Ranges from 100-1000 hours below 7°C depending on cultivar |
| Photoperiod response | Light perception genes modify flowering in response to day length |
| Temperature sensing | Vernalization pathway genes track cumulative cold exposure |
| Epigenetic regulation | Histone modifications silence DAM genes during winter |
| Bloom time variation | Genetic differences create 6-8 week span among cultivars |
| Climate adaptation | Low-chill alleles enabled expansion to subtropical regions |
5. Peaches and Skin Morphology: The Fuzz Factor in Evolution
The fuzzy skin covering most peaches serves multiple ecological functions. These tiny hairs, called trichomes, reflect ultraviolet radiation that could damage underlying tissues. They reduce water loss from the fruit surface by creating a boundary layer of still air. They also deter certain insects and fungal spores from establishing infections. This multi-functional trait provided adaptive advantages that natural selection reinforced.
Nectarines represent a naturally occurring mutation that eliminates peach fuzz. A single recessive gene controls this difference. The gene, identified as MYB25 or PpeMYB25, regulates trichome development across the fruit surface. Peaches with two functional copies of this gene develop normal fuzz. Nectarines carry two mutated copies that fail to initiate trichome formation. Heterozygous trees with one functional and one mutated copy produce fuzzy fruits, confirming the recessive inheritance pattern.
The genetic control of trichomes involves a complex regulatory network. MYB25 acts as a transcription factor that activates genes responsible for building trichome structures. These downstream genes produce proteins forming the hair shaft and secrete compounds that make the fuzz feel velvety. Mutations anywhere in this network can alter trichome density or structure. Some cultivars have sparse fuzz while others develop thick, almost woolly coatings.
Ecological studies suggest that trichome density responds to environmental pressure. Peach varieties from arid regions tend to have denser fuzz, supporting the water conservation hypothesis. Varieties from humid climates show reduced fuzz development. This correlation indicates that natural selection shaped trichome expression based on local moisture availability. Human selection then modified this trait further based on market preferences. Some consumers prefer smooth nectarines while others favor traditional fuzzy peaches. Both types persist because the genetic switch between them is simple and reversible.
Trichome Development and Function
| Aspect | Genetic and Ecological Details |
|---|---|
| MYB25 gene | Master regulator controlling entire trichome development program |
| Inheritance pattern | Single recessive locus explains nectarine mutation completely |
| Trichome density | Varies from 100 to 400 hairs per square centimeter |
| Water loss reduction | Fuzzy skin reduces transpiration by 15-25% versus smooth skin |
| UV protection | Trichomes reflect 30-40% of incident ultraviolet radiation |
| Pest deterrence | Creates physical barrier against small insects and mites |
| Cuticle interactions | Wax production genes work with MYB25 to form protective layer |
| Regional variation | Arid climate cultivars show 50% higher trichome density |
6. Peaches and Human Influence: Selective Breeding and Modern Genomics
Human cultivation transformed peaches more profoundly than natural selection alone could achieve. Over six thousand years, farmers selected trees producing larger, sweeter, more colorful fruits. This artificial selection created cultivars that would struggle to survive without human care. Modern peaches depend on grafting, irrigation, and pest management. Their seeds often fail to breed true, requiring vegetative propagation to maintain desirable traits.
Early breeding relied on phenotypic observation. Chinese cultivators noticed superior trees and propagated them through grafting. This process preserved favorable gene combinations that would have been broken apart by sexual reproduction. Persian and later European breeders continued this tradition, slowly accumulating beneficial mutations. Each generation selected a smaller proportion of trees for propagation, intensifying the genetic changes.
The genomic era revolutionized peach breeding. Complete genome sequences revealed which specific genes underlie important traits. Marker-assisted selection now allows breeders to identify seedlings carrying desired alleles before trees mature enough to fruit. This approach reduces breeding cycle time from seven years to three or four. CRISPR gene editing enables precise modifications impossible through conventional breeding. Researchers have used CRISPR to modify sweetness genes, disease resistance loci, and flowering time controls.
These modern tools raise important questions about genetic diversity conservation. Commercial peach production relies on a narrow genetic base derived from a small number of superior cultivars. This uniformity increases vulnerability to new pests or diseases. Researchers are responding by sequencing wild Prunus species and landraces from traditional growing regions. These genetic resources contain alleles lost during domestication. Reintroducing wild diversity while maintaining fruit quality represents a major challenge for future breeding programs. Success requires balancing productivity with resilience in ways that honor both the fruit’s evolutionary heritage and contemporary agricultural demands.
Modern Breeding Technologies and Applications
| Technology | Application in Peach Improvement |
|---|---|
| Genome sequencing | Completed 2013, enabled identification of all 27,852 genes |
| Marker-assisted selection | Reduces breeding time by 40% through seedling genotyping |
| CRISPR gene editing | Successfully modified disease resistance and fruit quality genes |
| Genomic selection | Predicts seedling performance from DNA markers alone |
| Wild germplasm utilization | Reintroduces disease resistance from P. davidiana and P. kansuensis |
| Transcriptome analysis | Identifies genes active during fruit development and ripening |
| Metabolomics integration | Links genetic variation to sugar and volatile compound profiles |
| Climate adaptation breeding | Develops low-chill cultivars for warming winters |
Conclusion: The Ongoing Genetic Symphony of Peaches
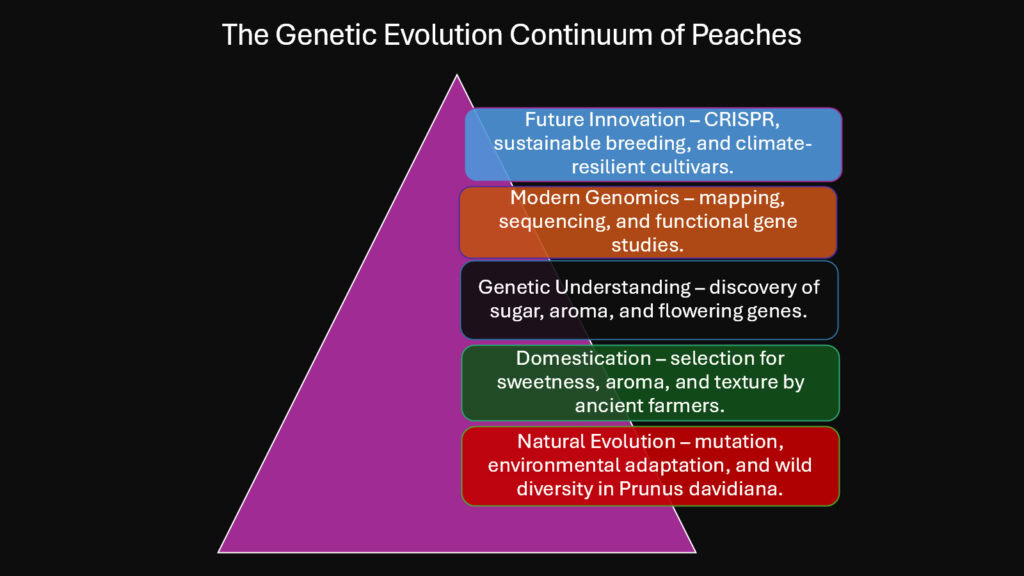
The story encoded in peach genomes blends natural evolution with human intention across eight millennia. Six genetic secrets illuminate this narrative. First, wild ancestors contributed resilience genes that persist in cultivated varieties. Second, sugar metabolism genes underwent intense selection that transformed taste. Third, volatile biosynthesis pathways diversified to create regional aroma signatures. Fourth, flowering time genes adapted to enable cultivation across varied climates. Fifth, trichome development genes shaped the fuzzy or smooth skin phenotypes. Sixth, human breeding accelerated genetic change beyond what nature alone would accomplish.
These secrets reveal peaches as living documents of biological and cultural history. Each bite contains molecules synthesized by enzymes whose genes bear traces of ancient selection. The fruit’s sweetness reflects Chinese farmers noticing superior trees six thousand years ago. Its fragrance carries signals once meant for seed dispersers but now calibrated to human preferences. Its flowering time embodies genetic solutions to climate challenges both past and present.
Looking forward, peach genetics promises continued discoveries. Climate warming demands new cultivars with altered chilling requirements and heat tolerance. Sustainable agriculture requires varieties resistant to diseases without heavy pesticide applications. Consumer preferences shift toward novel flavors and nutritional profiles. These challenges will be met through a deeper understanding of how genes build peaches from blossom to ripe fruit. Wild relatives waiting in mountain valleys and gene banks hold solutions encoded millions of years ago.
Modern tools can access this ancient diversity and recombine it in ways that ensure peaches remain viable, delicious, and genetically robust for generations ahead. The genetic symphony continues, each generation adding new notes to a melody that began when humans first tasted a fruit and imagined something better.
Future Directions of Genetic Research in Peaches
| Research Area | Current Focus | Expected Outcomes |
|---|---|---|
| Climate resilience | Identifying heat and drought tolerance genes from wild species | New cultivars maintaining productivity under 2-3°C warming |
| Disease resistance | Mining ancestral resistance loci lost during domestication | Reduced fungicide requirements by 40-60% |
| Nutritional enhancement | Modifying carotenoid and polyphenol biosynthesis pathways | Varieties with 2-3 times higher antioxidant content |
| Flavor optimization | Fine-tuning sugar-acid balance and volatile profiles | Regionally adapted taste matching consumer preferences |
| Extended shelf life | Reducing ethylene production and cell wall degradation | Fruits maintaining quality 5-7 days longer post-harvest |
| Low-chill breeding | Combining subtropical alleles for reduced dormancy | Expansion to regions with mild winters below 200 chill hours |
| Gene bank conservation | Sequencing and preserving 2000+ landraces and wild accessions | Safeguarding genetic diversity for future breeding needs |
| CRISPR applications | Precise editing of 3-5 genes simultaneously | Stacked traits combining disease resistance with quality |




