Table of Contents
Introduction: Pears – The Genetic Story Hidden in Their Cells

A pear sits in your hand, cool and smooth. Its shape seems simple enough. But inside every cell of that fruit lies a story millions of years old. The genome of this ordinary fruit holds clues about how plants adapt, survive, and change across time. Scientists who study pears aren’t just looking at lunch. They’re reading a molecular diary written in chromosomes and genes.
Pears belong to the Rosaceae family, a sprawling plant group that includes apples, roses, cherries, and strawberries. While roses captivate us with petals and apples dominate orchards worldwide, pears quietly carry their own remarkable genetic heritage. When researchers sequenced the pear genome in 2013, they opened a window into fruit evolution itself. The data revealed patterns of hybridization, gene duplication, and biochemical innovation that span continents and climates.
What makes pears particularly fascinating is their complexity. Their genome contains around 42,000 genes, more than humans possess. This genetic abundance arose through ancient duplication events, creating redundancy that became opportunity. Some duplicated genes evolved new functions while others maintained old ones. The result is a fruit with intricate sugar pathways, diverse aroma compounds, and adaptive resilience.
Six genetic secrets emerge from the pear genome, each revealing how evolution shaped this fruit. These secrets connect ancient ancestry to modern breeding, cellular chemistry to orchard survival, and wild species to cultivated varieties. Understanding them changes how we see not just pears, but the evolutionary forces that sculpt all living things.
Table 1: Comparative Genomic Features of Pears and Other Rosaceae Fruits
| Fruit Species | Chromosome Number | Genome Size (Mb) | Gene Count | Domestication Region | Key Genetic Trait |
|---|---|---|---|---|---|
| Pears (Pyrus communis) | 34 (2n=34) | 527-577 | ~42,000 | Europe, Asia | Whole genome duplication |
| Apple (Malus domestica) | 34 (2n=34) | 742 | ~57,000 | Central Asia | Ancient polyploidy event |
| Cherries (Prunus avium) | 16 (2n=16) | 338 | ~43,349 | Europe, West Asia | Compact genome structure |
| Peaches (Prunus persica) | 16 (2n=16) | 227 | ~27,852 | China | Model for Prunus genetics |
| Plums (Prunus domestica) | 48 (2n=48) | 450-600 | ~34,000 | Eurasia | Hexaploid hybrid origin |
| Strawberries (Fragaria × ananassa) | 56 (2n=56) | 698 | ~108,000 | Americas, Europe | Octoploid genome |
| Apricots (Prunus armeniaca) | 16 (2n=16) | 217 | ~28,000 | Central Asia | Drought tolerance genes |
| Raspberries (Rubus idaeus) | 14 (2n=14) | 224 | ~29,800 | Europe, Asia | High antioxidant pathways |
1. Pears and Their Rosaceae Heritage: The Shared Genetic Lineage
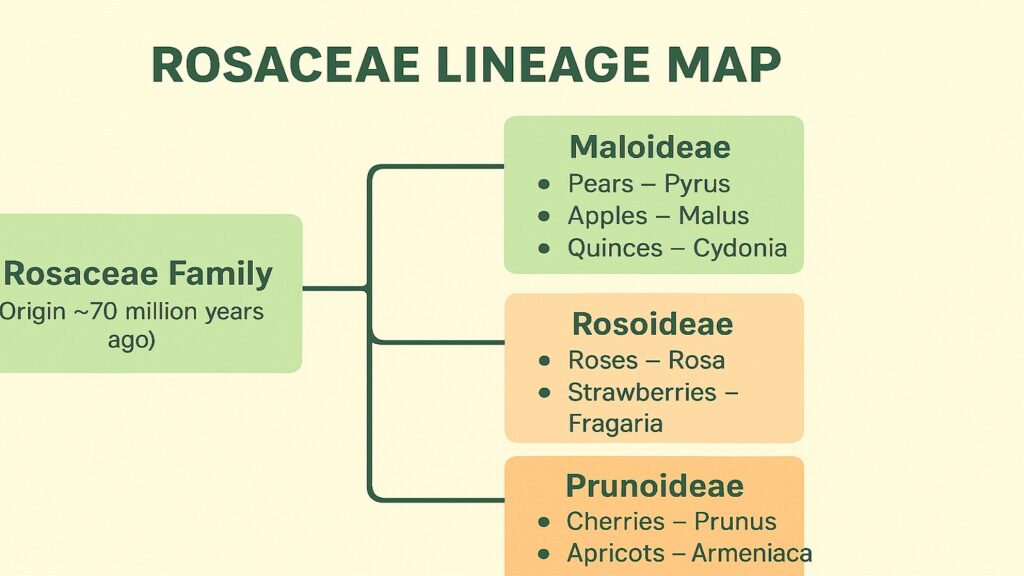
The Rosaceae family tree branches wide. Roses bloom in gardens while apples hang in orchards, yet they share molecular ties that stretch back roughly 100 million years. Pears sit on one of those branches, sharing a recent ancestor with apples that lived perhaps 50 to 60 million years ago. When continents drifted and climates shifted, this common ancestor diverged into separate genera: Pyrus for pears and Malus for apples.
Both pears and apples carry 17 pairs of chromosomes, giving them the same diploid number of 34. This similarity isn’t coincidence but inheritance from shared ancestry. Comparative genomics shows extensive chromosomal synteny between the two fruits, meaning large blocks of genes appear in the same order on corresponding chromosomes. Despite millions of years of separation, their genomes remain recognizable cousins.
The evolutionary history of Rosaceae involved dramatic genomic events. Researchers have identified an ancient whole genome duplication that occurred before the family diversified. This duplication effectively doubled every gene, creating genetic redundancy. Over time, many duplicated genes were lost, but others persisted and evolved new functions. This process, called subfunctionalization, allowed plants to develop specialized traits without losing essential functions.
Polyploidy played a crucial role in Rosaceae evolution. When genomes duplicate, plants gain raw material for innovation. Some Rosaceae members like strawberries became octoploid with eight copies of their base genome. Plums are hexaploid. Pears and apples, while diploid now, bear signatures of ancient polyploidy events in their gene arrangements and chromosome structure.
The split between pears and apples reflects adaptation to different ecological niches. Apples evolved characteristics suited to temperate forests with distinct seasons. Pears developed their own strategies, including distinctive fruit texture and ripening patterns. Their cells contain stone cells, specialized structures that give pears their characteristic gritty texture. The genes controlling these structures differentiated after the genera separated.
Quinces, another close relative in the tribe Maleae, complete this genetic triangle. While less commercially important than apple or pear, quince shares the same chromosome number and similar genome structure. Studies of gene flow between these species reveal occasional natural hybridization, though reproductive barriers generally keep them distinct.
Table 2: Evolutionary Relationships Within Rosaceae Subfamily Maloideae
| Genus | Common Name | Divergence Time (Million Years Ago) | Chromosome Number | Distinctive Evolutionary Feature |
|---|---|---|---|---|
| Pyrus | Pear | 50-60 from Malus | 2n=34 | Stone cell development genes |
| Malus | Apple | 50-60 from Pyrus | 2n=34 | Extended storage capability |
| Cydonia | Quince | 55-65 from Pyrus/Malus | 2n=34 | High pectin content genetics |
| Eriobotrya | Loquat | 40-50 from core Maloideae | 2n=34 | Subtropical adaptation |
| Chaenomeles | Flowering Quince | 60-70 from core group | 2n=34 | Ornamental flower genes |
2. Pears and the Power of Gene Duplication: Nature’s Genetic Experiment
Gene duplication creates possibility. When a gene copies itself within the genome, evolution gains freedom to experiment. One copy maintains the original function while the other can mutate, change, or develop entirely new roles. For pears, ancient duplication events produced a genome rich with genetic redundancy that became functional diversity.
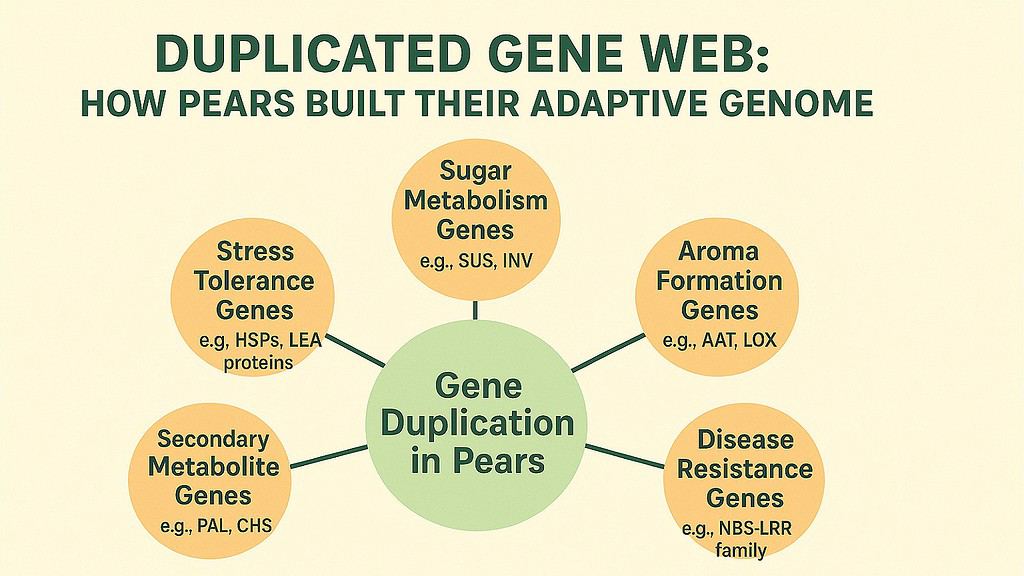
The pear genome shows evidence of multiple duplication events occurring at different times. The most ancient duplication coincides with the polyploidy event shared across Rosaceae. Later duplications occurred within the Pyrus lineage specifically. Researchers analyzing the genome found that roughly 40 percent of pear genes exist in duplicated pairs or families, each member showing varying degrees of divergence.
Sugar metabolism in pears illustrates how duplication enables specialization. Pears accumulate sugars differently than apples, preferring sorbitol as a transport sugar alongside sucrose. Multiple copies of sorbitol dehydrogenase genes allow pears to efficiently convert sorbitol to fructose in developing fruit. One copy might work primarily in leaves during photosynthesis, while another activates in fruit tissue during ripening. This division of labor emerged from an ancestral gene that duplicated and diverged.
Aroma formation depends heavily on duplicated gene families. Pears produce over 100 volatile compounds that contribute to their characteristic scent. Many of these compounds arise from the action of enzymes encoded by duplicated genes. The alcohol acyltransferase gene family, for instance, includes multiple members that create different ester compounds. Each ester contributes a specific note to the overall aroma profile, from floral to fruity to buttery.
Disease resistance genes show particularly extensive duplication. These genes, often called R genes, help plants recognize and respond to pathogens. The pear genome contains hundreds of R genes, many organized in clusters on chromosomes. This abundance provides genetic insurance. When a pathogen evolves to evade one resistance gene, duplicated copies with slightly different recognition capabilities may still mount an effective defense.
The molecular toolbox concept helps explain why duplication matters for evolution. Imagine a carpenter with only one hammer. If that hammer breaks, work stops. But with multiple hammers of different sizes and weights, the carpenter can handle diverse tasks and keep working if one tool fails. Duplicated genes function similarly, providing backup and enabling specialization simultaneously.
Not all duplicated genes persist or prove useful. Many accumulate mutations that destroy their function, becoming pseudogenes. The pear genome contains numerous pseudogenes, molecular fossils of ancient duplications that no longer serve active roles. These silent genes nonetheless provide evidence of evolutionary history, showing which genetic experiments succeeded and which failed.
Table 3: Major Gene Families Expanded Through Duplication in Pears
| Gene Family | Number of Copies | Primary Function | Evolutionary Advantage |
|---|---|---|---|
| Sorbitol Dehydrogenase (SDH) | 6-8 copies | Sugar metabolism and transport | Efficient carbohydrate partitioning |
| Alcohol Acyltransferase (AAT) | 12-15 copies | Ester and aroma production | Diverse volatile compound synthesis |
| Nucleotide-Binding Leucine-Rich Repeat (NLR) | 400+ copies | Disease recognition and resistance | Broad pathogen defense capability |
| Auxin Response Factors (ARF) | 23 copies | Growth hormone signaling | Fine-tuned developmental control |
| MYB Transcription Factors | 200+ copies | Gene expression regulation | Specialized metabolic responses |
| Expansin | 30+ copies | Cell wall modification | Fruit texture and growth patterns |
3. Pears and Hybridization: Blurring the Genetic Borders

Species boundaries sometimes blur in nature. While scientists categorize organisms into distinct species, plants often cross those boundaries through hybridization. Pears exemplify this genetic fluidity, with wild and cultivated species occasionally exchanging genes through natural or human-facilitated crosses.
European pears, classified as Pyrus communis, and Asian pears, known as Pyrus pyrifolia, represent distinct species with different characteristics. European pears have the familiar teardrop shape and buttery texture when ripe. Asian pears grow round and crisp, maintaining their firmness even when fully mature. Despite these differences, the two species can interbreed, producing fertile hybrid offspring.
Hybrid vigor, or heterosis, emerges when diverse genetic backgrounds combine. Hybrids between European and Asian pears often display enhanced traits compared to either parent. Some hybrids show improved cold hardiness inherited from European pears alongside the crisp texture of Asian pears. Others combine disease resistance from one parent with flavor characteristics from the other. This mixing of genetic material creates novel combinations that breeders can exploit.
Wild pear species contribute valuable genetic diversity to cultivated varieties. Species like Pyrus calleryana, native to China and Vietnam, possess strong resistance to fire blight, a devastating bacterial disease. Breeders have worked to transfer this resistance into commercial varieties through hybridization programs. The process takes years and multiple generations, but the genetic diversity present in wild populations makes it possible.
Natural hybridization occurs where different pear species grow in proximity. In Central Asia, multiple Pyrus species overlap in range, creating opportunities for spontaneous crosses. Some botanists believe that certain cultivated varieties arose from such natural hybrids in ancient times. Modern genetic analysis using molecular markers can trace these hybrid origins, revealing the complex ancestry of familiar cultivars.
Hybridization contributes to biodiversity within the genus Pyrus by generating genetic variation that natural selection can act upon. In changing environments, hybrid populations may contain individuals with novel trait combinations that prove advantageous. Over time, successful hybrids can establish as new lineages, expanding the evolutionary potential of the genus.
Reproductive barriers prevent unlimited hybridization. Different chromosome numbers, flowering time mismatches, and pollen-pistil incompatibility systems all limit which crosses can succeed. These barriers maintain species identity while allowing occasional gene flow. The balance between isolation and connection shapes pear diversity across geographic regions and ecological zones.
Table 4: Major Pear Species and Their Hybridization Potential
| Species | Common Name | Geographic Origin | Key Traits | Hybridization Frequency with P. communis |
|---|---|---|---|---|
| Pyrus communis | European Pear | Europe, Western Asia | Buttery texture, high quality | Self-species |
| Pyrus pyrifolia | Asian/Sand Pear | East Asia | Crisp texture, round fruit | Moderate, fertile hybrids |
| Pyrus bretschneideri | Chinese White Pear | Northern China | Cold hardy, sweet | Moderate, fertile hybrids |
| Pyrus ussuriensis | Ussurian Pear | Northeast Asia | Extreme cold tolerance | Low to moderate |
| Pyrus calleryana | Callery Pear | China, Vietnam | Fire blight resistance | Low, often sterile |
| Pyrus nivalis | Snow Pear | Southern Europe | Drought tolerance | Low to moderate |
4. Pears and Genetic Clues to Flavor: The Chemistry of Taste
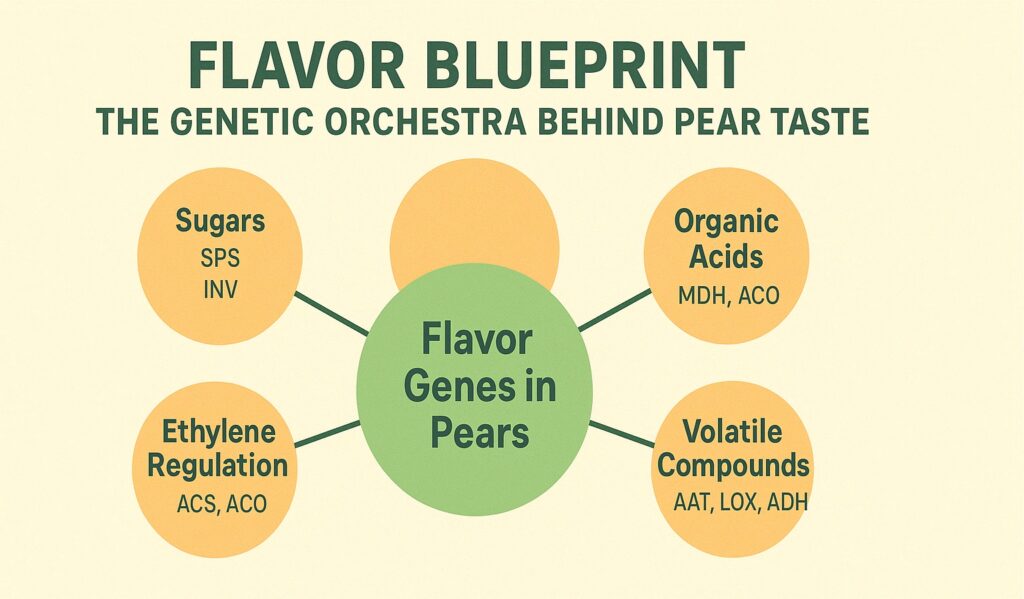
Flavor lives in molecules. When you bite into a ripe pear, your tongue detects sugars and acids while your nose captures volatile compounds drifting upward. Every sensation has a genetic basis, with specific genes controlling the synthesis, accumulation, and release of flavor molecules.
Sugar content determines perceived sweetness. Pears accumulate glucose, fructose, and sucrose in varying ratios. Genes encoding enzymes like sucrose synthase, invertase, and sorbitol dehydrogenase control sugar metabolism during fruit development. As a pear ripens, these enzymes break down starch reserves and convert sorbitol into sweeter sugars. The timing and intensity of enzyme expression, regulated by transcription factors, determines whether a variety tastes mildly sweet or intensely sugary.
Organic acids provide tartness that balances sweetness. Malic acid predominates in pears, though citric acid also appears in smaller amounts. The gene for phosphoenolpyruvate carboxylase initiates organic acid synthesis, while genes for acid transporters move these molecules into vacuoles where they accumulate. As fruit matures, acid levels typically decline while sugars increase, shifting the flavor profile toward sweetness.
Volatile compounds create aroma, the most complex aspect of pear flavor. Over 100 different volatiles contribute to the scent of a ripe pear, including esters, alcohols, aldehydes, and terpenes. The alcohol acyltransferase gene family produces esters with fruity, floral notes. Lipoxygenase genes generate green, grassy aldehydes. Each compound appears at specific concentrations, and the total blend creates the characteristic pear aroma.
Ethylene plays a central role in ripening and flavor development. This gaseous plant hormone triggers a cascade of changes that transform a hard, starchy fruit into something soft and sweet. Genes encoding ethylene biosynthesis enzymes, particularly ACC synthase and ACC oxidase, control ethylene production. Once ethylene synthesis begins, it activates ripening-related genes including those for cell wall degradation enzymes, sugar metabolism, and volatile production.
European pears exhibit climacteric ripening, meaning they can be harvested mature but unripe and will continue to ripen off the tree. This trait depends on ethylene signaling. Asian pears, in contrast, are non-climacteric and must ripen on the tree. The genetic differences between these ripening types involve mutations in ethylene receptor genes and downstream signaling components. Understanding these differences helps breeders develop varieties with specific ripening behaviors.
The ethylene receptor genes in pears include PcETR1, PcERS1, and others that detect ethylene and initiate cellular responses. Mutations in these genes can delay or prevent normal ripening. Some commercial varieties have been selected for altered ethylene perception, allowing longer storage without triggering premature softening.
Table 5: Key Genes Controlling Flavor Compounds in Pears
| Gene/Gene Family | Enzyme/Protein | Compound Produced | Sensory Contribution | Expression Pattern |
|---|---|---|---|---|
| Sucrose Synthase (SuSy) | Sucrose synthase | Sucrose, UDP-glucose | Sweetness | Increases during ripening |
| Sorbitol Dehydrogenase (SDH) | Sorbitol dehydrogenase | Fructose from sorbitol | Sweetness | High in mature fruit |
| Alcohol Acyltransferase (AAT) | Ester synthase | Hexyl acetate, butyl acetate | Fruity, floral aroma | Peak at full ripeness |
| Lipoxygenase (LOX) | Lipid oxidase | C6 aldehydes, alcohols | Green, grassy notes | Early fruit development |
| ACC Oxidase (ACO) | Ethylene synthase | Ethylene | Ripening trigger | Sharp increase at climacteric |
| Phosphoenolpyruvate Carboxylase (PEPC) | Carboxylase | Malic acid precursors | Tartness | High in young fruit |
5. Pears and Stress-Response Genes: Surviving Nature’s Challenges

Survival requires adaptation. Pear trees face drought in summer, frost in winter, and pathogens year-round. Their genomes encode defensive systems that detect stress and mount appropriate responses. These genetic defenses evolved through selective pressures operating across millions of years and diverse climates.
Heat-shock proteins exemplify stress response systems. These molecular chaperones prevent protein damage during temperature extremes. When a pear tree experiences heat stress, heat-shock protein genes activate rapidly, producing proteins that stabilize cellular structures. The pear genome contains multiple heat-shock protein gene families, each responding to different temperature thresholds and protecting different cellular compartments.
Cold tolerance genes help pear trees survive winter temperatures. Dehydrins, a class of proteins that protect cells during freezing, accumulate in pear tissues as temperatures drop. Cold-responsive transcription factors like CBF genes activate entire networks of protective genes. Some pear species native to northern regions possess more active cold response systems than southern species, reflecting their evolutionary history in harsh climates.
Drought stress triggers changes in gene expression that conserve water and maintain cellular function. Aquaporin genes encoding water channel proteins help regulate water movement across cell membranes. Transcription factors from the DREB family activate drought-responsive genes when soil moisture declines. Genes for proline synthesis increase, and this amino acid accumulates as an osmotic protectant, helping cells maintain turgor pressure.
Disease resistance involves both preformed defenses and inducible responses. The pear genome contains hundreds of resistance genes that recognize specific pathogen molecules. Upon recognition, these genes trigger defense responses including cell death at infection sites, production of antimicrobial compounds, and systemic acquired resistance that protects the whole plant.
Fire blight, caused by the bacterium Erwinia amylovora, devastates susceptible pear varieties. Resistant varieties possess functional resistance genes that recognize bacterial proteins and activate defenses quickly enough to halt infection. Researchers have mapped several fire blight resistance loci in pears, identifying genes that confer varying levels of protection.
Antioxidant enzyme genes protect cells from oxidative damage during stress. Superoxide dismutase, catalase, and peroxidase genes encode enzymes that neutralize reactive oxygen species generated during drought, heat, or pathogen attack. The pear genome contains multiple copies of these essential genes, ensuring robust antioxidant capacity.
Transcription factors coordinate stress responses by controlling multiple genes simultaneously. The pear genome includes large families of transcription factors like WRKY, NAC, and MYB proteins. Each family member recognizes specific DNA sequences in gene promoters, activating or repressing genes in response to environmental signals. This hierarchical control allows rapid, coordinated responses to changing conditions.
Table 6: Major Stress-Response Gene Families in Pears
| Gene Family | Number of Members | Stress Type | Molecular Function | Evolutionary Conservation |
|---|---|---|---|---|
| Heat-Shock Proteins (HSP) | 50+ | Heat, cold | Protein folding, protection | Ancient, across all life |
| Dehydrins (DHN) | 8-12 | Cold, drought | Membrane stabilization | Plant-specific, widespread |
| CBF/DREB Transcription Factors | 15-20 | Cold, drought | Cold gene activation | Plant-specific, conserved |
| NLR Resistance Genes | 400+ | Pathogens | Pathogen recognition | Plant-specific, rapidly evolving |
| Superoxide Dismutase (SOD) | 8-10 | Oxidative stress | ROS neutralization | Ancient, all organisms |
| Aquaporins (PIP, TIP) | 35-40 | Drought | Water transport | Ancient, conserved structure |
6. Pears and the Future of Genetic Research: Mapping Tomorrow’s Orchards
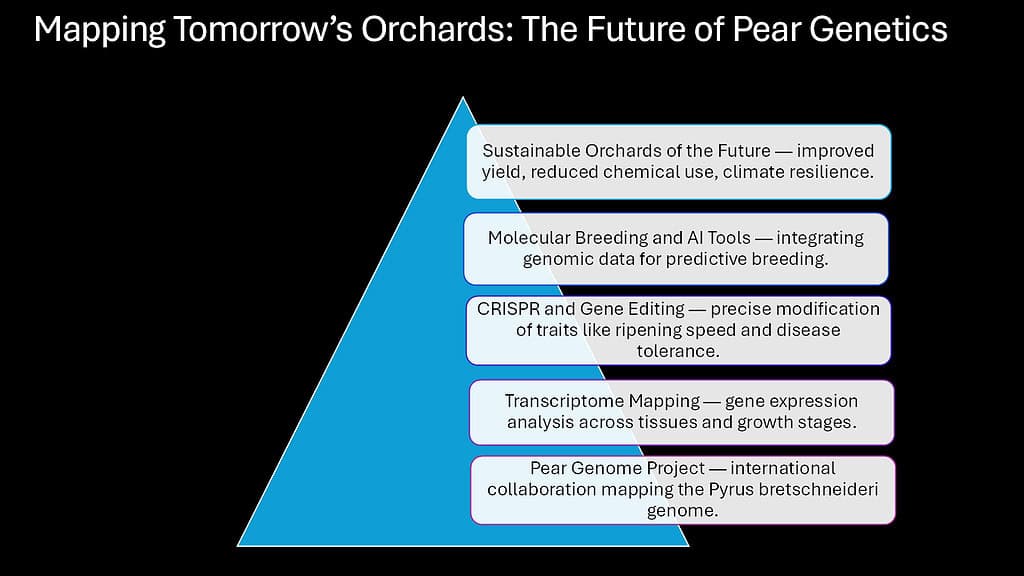
Genomic research continues to transform how we understand and improve pears. The complete genome sequence published in 2013 provided a foundation, but ongoing research adds layers of detail and functional understanding. New technologies enable precise genetic modifications and accelerate breeding programs that once required decades.
CRISPR-based gene editing offers unprecedented precision for modifying pear genetics. This technology allows researchers to target specific genes and make exact changes without introducing foreign DNA. Scientists have successfully used CRISPR to edit genes controlling fire blight resistance, fruit ripening, and storage characteristics in pear cells. While regulatory and public acceptance challenges remain, the technology demonstrates potential for creating improved varieties.
Transcriptome mapping reveals which genes activate in different tissues and developmental stages. By sequencing RNA from pear flowers, leaves, and fruit at various ripening stages, researchers create expression atlases showing gene activity patterns. These maps help identify genes controlling specific traits and reveal regulatory networks coordinating complex processes.
Molecular breeding uses genetic markers to select plants with desired traits. Instead of waiting years for trees to mature and fruit, breeders can test seedlings using DNA markers linked to traits like disease resistance or fruit quality. This marker-assisted selection accelerates breeding programs, reducing the time needed to develop new varieties from 15-20 years to potentially less than a decade.
The Pear Genome Project represents international collaboration among researchers in China, Europe, and the United States. This consortium sequences genomes from diverse pear species, creating a pan-genome resource that captures genetic variation across the genus. Understanding this diversity helps breeders access genes that might improve cultivated varieties.
Genetic research supports sustainable agriculture by enabling crops that require fewer chemical inputs. Disease-resistant varieties reduce fungicide and bactericide applications. Drought-tolerant trees maintain productivity with less irrigation. Understanding the genetic basis of these traits allows their targeted improvement through breeding or genetic modification.
Climate change presents new challenges for pear cultivation as temperature patterns shift and growing seasons change. Genomic research identifies genes controlling adaptation to new conditions. Some genes might extend cold hardiness for northern orchards experiencing warmer winters, while others could enhance heat tolerance for southern regions facing hotter summers.
Flavor improvement remains a breeding priority, and genetic research provides tools for success. By identifying genes controlling specific aroma compounds or sugar types, breeders can pyramid multiple favorable alleles into single varieties. The goal is creating pears that satisfy consumer preferences while maintaining productivity and disease resistance.
Table 7: Current Genetic Research Directions for Pear Improvement
| Research Area | Technology Used | Target Traits | Potential Impact | Current Status |
|---|---|---|---|---|
| Gene Editing | CRISPR-Cas9 | Fire blight resistance, storage life | Reduced disease loss, extended marketing | Laboratory trials successful |
| Marker-Assisted Selection | SNP genotyping | Multiple quality and resistance traits | Faster breeding cycles | Widely implemented |
| Transcriptome Analysis | RNA-sequencing | Gene regulatory networks | Understanding trait control | Ongoing, expanding databases |
| Pan-Genome Development | Multiple genome sequencing | Capture wild species diversity | Access to novel alleles | Initial assemblies complete |
| Metabolomics Integration | Mass spectrometry with genomics | Flavor compound optimization | Consumer-preferred varieties | Research stage |
| Climate Adaptation | Population genomics | Heat and drought tolerance | Sustainable production | Identifying candidate genes |
Conclusion: Pears: The Genome That Connects Past and Future

The story written in pear genomes spans deep time. Fifty million years ago, an ancestor split into lineages that became apples and pears. Gene duplication events created genetic abundance from which natural selection could choose. Hybridization blurred boundaries between species while maintaining diversity. Genes encoding flavors evolved to attract animals that would disperse seeds. Stress-response systems developed to survive harsh seasons and hungry pathogens.
Six genetic secrets emerge from studying pears. First, their Rosaceae heritage connects them to a diverse plant family through shared chromosomal structure and ancient polyploidy. Second, gene duplication provided genetic raw material that enabled specialization in sugar metabolism, aroma production, and disease resistance. Third, hybridization between species created novel genetic combinations and enhanced biodiversity within the genus.
Fourth, specific genes control the chemistry of flavor, from sugar accumulation to volatile compound synthesis to ripening signals. Fifth, stress-response genes evolved to help pears survive environmental challenges across their geographic range. Sixth, modern genomic research tools are mapping these genetic systems with increasing precision, enabling improvements in breeding and cultivation.
Understanding the pear genome reveals evolution itself in action. We see how random mutations become meaningful variations, how duplicated genes diverge into specialized functions, how populations adapt to local conditions through selection on favorable alleles. The genome is both history book and instruction manual, recording what happened and specifying what happens now.
Decoding this genomic story creates practical benefits. Breeders develop varieties with better flavor, stronger disease resistance, and improved climate resilience. Farmers grow trees that produce more reliably with fewer inputs. Consumers enjoy fruit that tastes better and stays fresh longer. These improvements arise from understanding the molecular mechanisms encoded in DNA.
The pear genome also reminds us that all organisms carry such stories. Every plant, animal, and microbe evolved through similar processes of mutation, duplication, and selection. The particular details differ, but the fundamental mechanisms unite all life. In studying pears, we study ourselves, learning about the evolutionary forces that shaped human genomes alongside those of the plants we cultivate.
Tomorrow’s orchards will grow from today’s genetic knowledge. As climate shifts and challenges evolve, the genetic diversity preserved in wild pear populations and cultivated varieties becomes increasingly valuable. Understanding which genes matter and how they function allows us to steward this diversity wisely, maintaining options for future adaptation.
The pear sits again in your hand, no longer quite so simple. Behind its smooth skin lies molecular complexity that took millions of years to evolve. The sweetness you taste when biting through that skin represents countless genetic decisions about sugar synthesis and transport. The aroma rising to your nose reflects a hundred volatile compounds produced by duplicated gene families. The resistance to diseases you never see protecting the fruit came from defensive genes honed by ancient selection pressures.
Pears embody both where life has been and where it might go, connecting botanical past to agricultural future through the elegant language of genetics.
Table 8: Summary of Six Genetic Secrets Revealed by Pear Genomics
| Genetic Secret | Key Discovery | Molecular Mechanism | Evolutionary Significance | Applied Benefit |
|---|---|---|---|---|
| Rosaceae Heritage | Shared ancestry with apples | Chromosomal synteny, ancient polyploidy | Family-level diversification | Cross-species knowledge transfer |
| Gene Duplication | Extensive duplicated gene families | Whole genome and segmental duplications | Functional specialization | Enhanced trait flexibility |
| Hybridization | Fertile crosses between species | Shared chromosome structure | Biodiversity maintenance | Hybrid vigor utilization |
| Flavor Genetics | Specific genes for taste compounds | Metabolic pathway genes | Seed dispersal adaptation | Targeted flavor breeding |
| Stress Response | Complex defense gene networks | Transcription factor cascades | Environmental adaptation | Climate-resilient varieties |
| Future Research | CRISPR and molecular breeding | Precise genetic modification | Continued evolution | Accelerated improvement |




